In this blog, we’ll help you in learning how to draw a lewis dot structure in the easiest and most precise way. Read on to know what these structure and learn about some of its best examples.
For more such chemistry based topics, also read our blog:-
How Many States of Matter are There Around US
What is a Lewis Dot Structure?
A Lewis Dot Structure is the diagrammatic representation of the bonding between the atoms of a molecule and the lone pair of electrons present in it. It is also known as electron dot structure/ Lewis dot diagram, Lewis dot formulas, Electron dot structure, or Lewis Electron dot structure (LEDs), respectively. The Lewis structure is named after Gilbert N. Lewis, who introduced it in his article Atom and molecule in 1916.
Purpose of drawing a Lewis Dot Structure:
- It helps in predicting the quantity and types of bond formed around an atom, ion, or molecule.
- Lewis structure helps in deriving the geometry of the molecules.
- Lewis structures back up the concepts of general chemistry such as chemical bonding, resonance, electron pair repulsion theory, etc.
- It initiates the prediction of the polarity of the molecules and understanding of reaction mechanisms.
Students often find the structures of chemistry as complex networks. However, Lewis Dot Structures are a simple representation of bod shown by driving lines amidst the valence electrons. To better understand how to form these structures, one ought to follow simple steps. You should follow the step-wise sequence to gain results.
Steps to draw a Lewis Electron Dot Structure:
Step 1: Find and count the total valence electrons
Valence electrons are the total number of electrons residing in the outermost shell or valence shell of the electronic configuration of an atom. These valence electrons act as the base structure for the Lewis dot structure. To form the initial structure, the total number of valence electrons, the initial structure can be formed.
The total number of valence electrons can be derived either through the group in which it is present or by writing the electronic configuration of the same.
Step 2: Find the number of participating electrons
These are the total number of electrons used for the stabilization of an atom. For instance, all the atoms require a particular number of electrons to complete their outermost shell and acquire a stable electronic configuration.
This rule states that each atom needs the number of electrons to an extent, to gain stable configuration concerning the nearest noble gas is called the Octet Rule.
Step 3: Derive the number of bonds in the molecule.
Each participatory electron from the atoms forms an electron pair, which helps in the formation of covalent bonds. By subtracting the electrons needed for the stabilization of an atom from the total valence electrons, gives off the number of electrons needed to complete the octet.
Each bond requires two electrons for its formation, so the number of bonds is equal to half the number of electrons needed.
In short: Step 2- Step 1/ 2 gives you the number of bonds in the molecule.
Step 4: Select a central atom
The central atom in a Lewis Dot Structure is the least electronegative of the atom with the highest valence.
Electronegativity is the tendency of an element to gain one or more electrons, to attain a stable configuration. It results in polarity. Electronegativity increases from left to right across the period (as the size of an atom decreases) and decreases top to bottom down the group (as the size of an atom increases).
The reason being, an increase in the size of the atom, distances the bond electrons from the nucleus of the atom. Hence, the nucleus of an atom will witness less pull on the electrons.
This decrease in the pull would help in the selection of an apt central atom, which leads to the formation of a stable Electron Dot Structure.
Important points to remember:
- Central atoms should make more than one single bond around it.
- Group 1 elements cannot have more than 2 electrons, due to the presence of only 1s orbitals in their configurations.
- Period 2 elements cannot have more than 8 electrons around the central atom; Due to the lack of empty d orbitals. Thus, fail in attaining expanded octet.
- H and F cannot act as the central atom, as they need only one electron to complete their respective duplet and octet. They make only single bonds with other elements.
Step 5: Prepare the skeleton of the structure
Draw a connected network through straight lines between the central atom and the surrounding atoms. The central atom can connect to four other atoms only. This set-up would help in the formation of the final Lewis Dot Structure.
The formal charge refers to the charge assigned to an atom in a molecule or an ion. It marks the difference in the total valence electrons and the number of electrons participating in the formation of a Lewis Dot Structure.
These charges are gained by an atom when it uses extra electrons after the complete utilization of the pre-existent valence electrons.
Remember: In a covalent bond, each atom gives one electron for the formation of the bond. Due to which for each lone pair of electrons, it uses a pair of electrons.
If the electrons around an atom are more than the valence electrons; it acquires a formal negative charge. Whereas, if it is less than the valence electrons; it acquires a formal positive charge.
Formal charge = valence electrons –Bond pair electrons and lone pair electrons in the Lewis dot structure) or
= Valence electrons – ½ electrons as bond pairs – electrons as lone pairs
Or
= Group No – No of bonds – 2*No lone pairs
Step 7: Complete the structure
The last step is to put the lone pairs of electrons around the central atom. These lone pairs are the left out electrons of the valence shell. Thus, the final structure is drawn with a central atom connected to the other atoms through straight lines.
Example of Lewis Electron Dot Structure (step-by-step)
Lewis Dot Structure of an atom
Lewis electron dot structure of Carbon
Electronic configuration of Carbon: 1s22s22p2
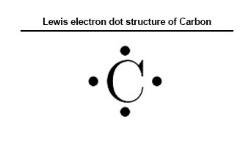
- Step 1:Find and count the total valence electrons.
Total valence electrons= 4
- Step 2: Find the many participating electrons.
Participating atoms= 8
- Step 3: Derive the several bonds in the molecule.
No. of bonds= 8-4 = 4\2= 2
- Step 4: Select a central atom.
Central atom= Carbon
- Step 5: Prepare the skeleton of the structure.
- Step 6: Attain complete octet.
Formal charge = 4-2=2
- Step 7: Complete the structure
Other Lewis dot structures
Lewis electron dot structure of Iodine
Electronic configuration of Iodine: 4d105s25p5

Lewis electron dot structure of Potassium
Electronic configuration of Potassium: 4s1

Lewis Dot Structure of mono-atomic ions
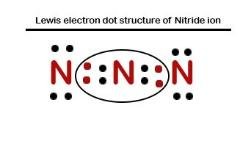
Lewis electron dot structure of Nitride ion
Electronic dot structure of Nitride Ion:
Electronic configuration for nitrogen =1s22s22p3
In order to complete the octet rule, nitrogen takes three electrons and attain -3 charge. So.N−3 = 1s22s22p6
Lewis electron dot structure of Sulfide ion
Electronic dot structure of Sulfide Ion:
Electronic configuration of Sulfide ion= 1s22s22p63s23p4
In order to complete the octet rule, Sulfide ions take two electrons and attain -2 charge. So S2− = 1s22s22p63s23p6

Lewis Dot structure of polyatomic ions and molecules
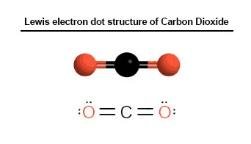
Lewis electron dot structure of Carbon Dioxide
Electronic configuration of Carbon Dioxide:
The electronic configuration of carbon = [He] 2s2 2p2
The electronic configuration of oxygen = [He] 2s2 2p4
To complete the octet it needs- oxygen atoms=2 and Carbon atom =1
Lewis electron dot structure of Hydrogen Sulfide
Electronic configuration of Hydrogen Sulfide:
The electronic configuration of Hydrogen: 1s1
The electronic configuration of Sulfur: 3s² 3p⁴
To complete the octet it needs- Hydrogen atoms=2 and Sulfur atom-=1

Lewis electron dot structure of Ammonium ion
Electronic configuration of Ammonium ion:
The electronic configuration of Nitrogen atom:
The electronic configuration of Hydrogen: 1s1
To complete the octet it needs, Nitrogen atom=1 and Hydrogen atom=4



